Many job tasks performed in the research laboratories place workers at risk for developing musculoskeletal disorders. This tutorial was designed to provide you with information to help your minimize risk.
Laboratory Ergonomics Tutorial
This tutorial is set up so you can move through the different sections in the way you’re most comfortable.If you choose to review the information sequentially, simply click through each panel in order. Otherwise, you can jump around each sections based on topic.
-
A Guide to Laboratory Ergonomics

Many job tasks performed in the research laboratories place workers at risk for developing musculoskeletal disorders. Potentially hazardous activities include the use of equipment such as pipettes, microscopes, microtomes, centrifuges, flow cytometers, cryostats, and computers. Prolonged and awkward postures, excessive reaching, lifting and repetitive motions may contribute to the onset of discomfort and eventual injury.
Standing and working in awkward positions in laboratory hoods and biosafety cabinets can also present ergonomic problems.
The following tips are offered to help you reduce your risk of acquiring musculoskeletal disorders (MSDs). These disorders develop over time and occur when muscles and joints are stressed, tendons are inflamed, nerves are pinched and bloodflow is restricted. By becoming familiar with how to control laboratory ergonomic risk factors, you can improve your comfort, productivity, and job satisfaction while lowering your chances for occupational injuries. Follow the guidelines below to learn more about ergonomics in the laboratory.
Ergonomic laboratory equipment is available through the University of Michigan Purchasing Department vendor contracts.
-
Be Aware of Your Posture
Maintain the three normal curves of the spine as much as possible, especially when maintaining static postures, bending and lifting. Bending your knees and keeping the inward curve in the low back will help reduce back strain.
-
Wear shoes with good support and cushioning if your work requires a lot of standing or walking.
-
If standing in one spot for long periods, anti-fatigue mats can help redistribute weight. Resting one foot on a small (4"-6") platform while standing, then shifting to the other foot, can help to relieve low back strain.

Ergo: Rest on a platform or box

Ergo: Use an anti-fatigue mat
-
If your feet dangle when you sit back in the chair or stool, adjust the footring or footrest so that your feet are supported.
-
If your stool lacks back support, you can tilt the seat forward or use a seat wedge to position the back and the pelvis in a more natural posture.
-
Try to avoid spending long periods looking down while reading. Use a copy holder to elevate materials.
-
-
Repetitive Pipetting
Pipetting is one of the most common tasks performed in the research laboratory. It involves several ergonomic stressors of the wrist, arms, and shoulders. The majority of these stressors are caused by excessive thumb force, repetition, and awkward postures. The following are recommendations for ergonomics hazards associated with the use of pipettes:
-
Use pipettes with newer trigger mechanisms requiring less force to activate, use the pointer finger to aspirate, and the thumb to dispense.

-
Use pipettes that fit comfortably in your hand.
-
For tasks such as mixing or aliquotting, use an electronic pipettor with mixing functions. They are programmable and reduce the need for excessive thumb force and repetitive motion.
-
Use a multichannel pipettor for large aliquotting tasks.

-
Use thin-walled pipette tips that fit correctly and are easy to eject.
-
Clean pipettors regularly. This reduces “sticking” and improves work quality.
-
Keep samples and instruments within easy reach.
-
Use only the force necessary to apply tips and operate the pipette.
-
Work at a cut-out in the lab bench when you can. This will allow you to work as close to the bench as possible and to sit back in the chair if you are seated.
-
Adjust the workstation so you don’t have to work with arms in an elevated position. Work with arms close to the body.
-
Keep head and shoulders in a neutral position (bent forward no more than 30 degrees).
-
Rotate pipetting activities between laboratory tasks. Change hands, if possible, and spread pipetting tasks among people.
-
Use an adjustable stool or chair when sitting at the lab bench so you will be working at the proper worksurface height for the work you are doing.
-
If it is necessary to stand for long periods of time during pipetting, use an anti-fatigue mat.
-
Take short, frequent opportunities (2-3 minutes for every 20-30 minutes) to stretch and perform mild hand exercises while pipetting to reduce muscle strain and fatigue.
-
-
Computer Workstation
Many researchers spend 50% or more of their day entering data with their keyboard and mouse resting on a lab bench. Lab benches can be too high, and require the researcher to elevate the arms and excessively deviate the wrists while inputting data. Depending on the location of the mouse, awkward reaches and manipulations of the mouse with bent wrists may occur. The following are recommended for control of ergonomic hazards associated with the use of computers in the lab:
-
Position the mouse beside the keyboard.
-
Replacing larger traditional monitors with flat panel monitors can increase valuable workspace
-
Fully adjustable seating will accommodate variations in body types of staff.
-
Place monitors at approximately arms’ length away from the body. The best distance from the monitor is when characters can be seen clearly without leaning forward.

No-No: Strain to back, shoulder and neck

Ergo: Less strain overall
-
Place the monitor so the top of the screen is at about eye level or slightly below. This allows the eyes to naturally gravitate toward the center of the screen and will keep the cervical spine in its natural curve.
-
Use a document holder placed next to and in the same plane as the computer screen.
-
Use footrests where possible in order to allow for changing leg positions throughout the day.
-
Take short, frequent opportunities to alternate your posture to stretch and move around to reduce muscle tension and fatigue.
-
Alternate work tasks to reduce muscle strain and fatigue. For example, move from keyboard to pipetting or other job tasks as your muscles feel stressed.
-
-
Microscopy
When designing the microscope workstation, the dimensions of those who will use the workstation must be considered. Since laboratory personnel come in many different shapes and sizes, the workstation should be easily adjustable. The following are recommendations for controlling ergonomic hazards associated with the use of microscopes:
-
Do not use a microscope for more than five continuous hours per day. Spread the use out over an entire workday, avoiding long, uninterrupted periods of microscope work.
-
Try pulling the microscope toward the edge of the worksurface to allow for a more upright posture.
-
Try elevating the microscope and placing it at an angle so you can look directly into the eyepiece with your head and neck in a more natural upright position. This can help you to be in a more neutral posture and reduce rounding of the shoulders and neck.
-
Keep your neck in a neutral posture and avoid extending the chin forward when using the microscope. Adjust the height of the scope or the chair so that you do not need to strain.

No-No: A bent neck contributes to neck strain
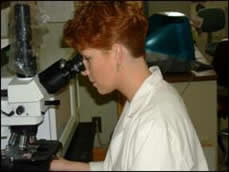
Ergo: Maintain a neutral posture
-
If available, use a cut-out work table. This allows you to get closer to your work and comfortably reach to the adjust knobs as well as providing an area for forearm support.
-
Use armrests to support your forearms while using adjustment knobs on the microscope.
-
Maintain natural spinal curves. Rather than bending from the waist to work at the microscope, bend forward from the hips.
-
If available, use an ergonomically adjustable chair that provides adequate back support, along with seat height and seat angle adjustability.
-
Provide height appropriate footrests or footrings on stools to avoid pressure to the backs of thighs while seated.

No-No: Inadequate legroom

Ergo: Shoulders are not stressed when items are close
-
Make sure there is adequate room under the work surface to pull your chair up to the ocular(s).
-
A sit-stand seat is recommended for areas where there is restricted legroom.
-
Take short, frequent opportunities to alternate your posture to stretch and move around to reduce muscle tension and fatigue.
-
Reduce eye fatigue by looking away from the microscope every 20 minutes and blink frequently.
-
Use television systems where possible to eliminate the use of binocular eyepieces.
-
-
Biosafety Cabinets and Laboratory Workbenches
Biosafety cabinets and laboratory workbenches present similar ergonomic hazards which are mostly due to the lack of adjustability and leg room. The following are guidelines to control these hazards:
-
If available, use an ergonomically designed chair that provides adequate back support, along with seat height and seat angle adjustability. Use the floor or footrests to support legs while seated.
-
If available, factory-applied movable armrests should be installed external to the cabinet or edge of the workbench to provide support for the arms and still maintain the required airflow. This reduces contact stress by increasing the surface area that comes into contact with the forearm and therefore, reduces the chances of impinging nerves, tendons, and blood vessels. If applying closed-cell padding to the biosafety cabinet, make sure the material can be properly decontaminated.
-
Remove drawers, supplies, and refrigerators from under the workbenches and cabinet doors from under the biosafety cabinets. This increases legroom and allows you to get closer to your work.
-
Use a turntable to store equipment near the worker. This reduces excessive reaching and twisting which places an increased load on the back and can lead to shoulder strain.
-
Position materials in the cabinet and on the bench top as close as possible to you to avoid extended reaching without compromising containment of the cabinet.
-
Use shoes with adequate cushion and/or anti-fatigue mats if you stand for long periods of time.
-
Take short, frequent opportunities to alternate your posture to stretch and move around to reduce muscle tension and fatigue.
-
New biosafety cabinets that incorporate the features below may be available and should be used if available:
-
A perforated front grill reduced by 1”-2” to bring the work platform closer to you.
-
Adjustable height (electric or hydraulic lift).
-
Non-glare glass on the sash and/or adjustable plexiglass barriers.
-
A platform configuration with “wells” for placement of tall containers.
-
-
Micro-Manipulation and Fine Motor Skills
Many laboratory procedures require repetitive use of the extensor and flexor muscles of the fingers and wrists. For example, removing caps and screw-off lids from vials, reaching into bins and the use of forceps all require the use of these small muscle groups and may result in awkward postures.


Prolonged repetitive fine motor tasks can cause discomfort in hands and wrists.
The following are recommended for control of ergonomic hazards associated with micro-manipulation techniques:
-
If feasible for your work, use plastic vials with fewer threads. This will reduce twisting motions during capping and uncapping.
-
Use small pieces of foam to prevent soreness on the fingertips, where fingers and forceps touch. This will distribute the force over a greater surface area, thus reducing the compressive forces on the soft tissue.
-
Practice using the forceps between the 1st and 2nd digits instead of using the thumb and 1st digit. Then try alternating between the two positions to reduce the use of the thumb. Many tasks within the laboratory require repetitive motion of the thumb.
-
Tilt storage bins toward you to reduce wrist flexion while reaching for supplies.
-
Alternate work tasks, take mini breaks, and perform mild hand stretches and exercises to reduce muscle strain and fatigue.
-
-
Microtome & Cryostat Work
The following guidelines are recommended for control of ergonomic hazards associated with the use of a microtome or cryostat:
-
Adjust the workstation to keep arms closer to the body.
-
Apply padding to the front edge of the work surface to protect forearms from contact stress which inhibits bloodflow.

No-No: Hard and sharp edges can damage soft tissue

Ergo: Soft edging to protect forearms
-
Retrofit the existing handle with an adapter that will allow you to use the handwheel in a pistol grip position. This will alleviate repetitive wrist flexion and extension.

Manual operation

Automation relieves shoulders
-
Consider using an automatic foot operated cryostat when frequent cryosection is performed.
-
Avoid placing utensils such as forceps inside the cryostat.
-
Use an adjustable chair.
-
Alternate work tasks, take mini breaks, and perform mild stretches and exercises to reduce muscle strain and fatigue.
-
-
Flow Cytometers
The use of a flow cytometer requires frequent lateral bending, neck and back flexion, and extended reaching with the arm. This is due to the receiving port being located on the bottom of the flow cytometer. The operator may need to sit in awkward positions in order to see the controls. The following are guidelines for control of ergonomic hazards associated with use of a flow cytometer:
-
If needed to work in neutral posture, raise the flow cytometer by placing a block between the flow cytometer and the workbench. When droplet exposure is a concern, a face shield and safety goggles are needed to protect your face. Ensure that these are properly positioned.
-
If possible, use an electric or hydraulic adjustable table. Each individual should be able to adjust the flow cytometer to the height that is most comfortable.
-
Use an adjustable chair, if available.
-
Place the top of the monitor so the top of the screen is at approximately eye level or slightly below.
-
-
Glove Boxes
Working in glove boxes or anaerobic chambers requires extended static loading on the shoulders. Extending the arms for more than a couple of minutes can become exhausting. In addition to static loading and frequent side reaching, the thick gloves can also cause the user to over-compensate on grip strength. Where possible, the following controls are recommended for ergonomic hazards associated with using a glove box:
-
Move all needed materials for the experiment from the side chamber to the main chamber at one time to reduce the amount of side reaching.
-
Use highly absorbent hand powder for glove comfort.
-
Expand the job (periodically perform other activities) to avoid long continuous use of glove boxes.
-
Use padded shoes and/or anti-fatigue matting if standing for long periods of time.
-
If necessary, use a sit-stand seat to alleviate stress on the low back.
-
Alternate work tasks, take mini breaks, and perform stretching exercises to relieve muscle stress from the shoulders.

-
-
Centrifuges
Centrifuge rotors present a unique lifting hazard in the laboratory. Centrifuge rotors can weigh up to 35 pounds and are awkward in shape. The following are recommended for control of ergonomic hazards associated with lifting centrifuge rotors:
-
If possible, use a second person to assist with lifting and removing the rotors.
-
Use a cart to transport rotors.
-
Consider purchasing equipment from vendors that manufacture lighter weight rotors.

-
-
Overhead Lifting
Due to limited space in the laboratory, many laboratory workers must store equipment and supplies on overhead shelves. The following are recommended for control of ergonomic hazards associated with overhead lifting:
-
Store heavy objects on shelves below shoulder height whenever possible.
-
Use a stable footstool or stepladder to reach objects that are stored on higher shelves.
-
Avoid asymmetrical lifting (twisting while lifting). The object to be lifted should be directly in front of the worker.
-
Use rotating carousels or turntables to store materials close to the worker. This reduces excessive reaching for objects.
-
-
Additional Resources
The following resources are available for more information about ergonomics:
Document Resources
-
Eastman Kodak Company. (1983). Ergonomic Design for People at Work — Volume 1. New York: John Wiley & Sons, Inc.
-
Eastman Kodak Company. (1986). Ergonomic Design for People at Work — Volume 2. New York: John Wiley & Sons, Inc.
-
Humantech, Inc. (1998). Applied Office Ergonomics Manual. Ann Arbor, MI
-
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH (1997).Elements of Ergonomics Programs. Cincinnati, OH
-
U.S. Department of Labor, Occupational Safety & Health Administration, Ergonomic Information